
Gas Absorption (“scrubbing”) is the process of removing a gas solute from a gas mixture by dissolving it with an absorbent liquid, usually referred to as scrubbing liquid or solvent. As the solvent comes in contact with the gas mixture, select components of the gas mixture dissolve — effectively separating the mixture.
In gas absorption, an appropriately chosen solvent must be relatively pure and has low volatility. In a gas scrubber, a closed space/container is filled partially with the solvent while letting the gas fill the remaining space causing contact between the two phases. As long as the gas dissolves only in the solvent and the solvent is not volatile, mass transfer of components from gas to liquid will occur.
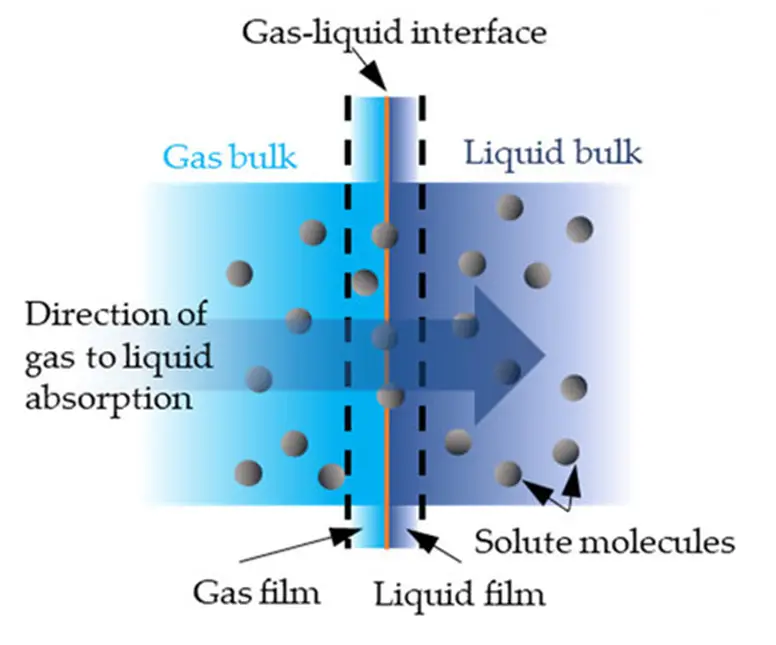
Temperature affects the gas-liquid mass transfer since only a specific amount of gas components will be dissolved by the liquid at a certain temperature. This amount of gas dissolved in the liquid is called equilibrium solubility and is expressed in units of concentration or composition.
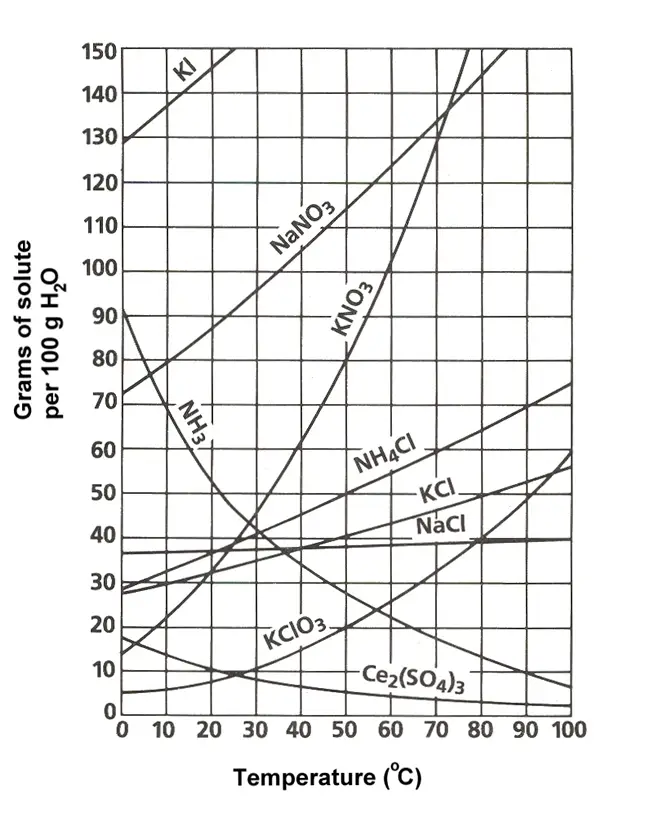
However, a significant increase in temperature may cause gas to escape from the liquid. In this case, absorption can be enhanced by adjusting the gas amount or gas pressure and determining how much gas is dissolved in the liquid.
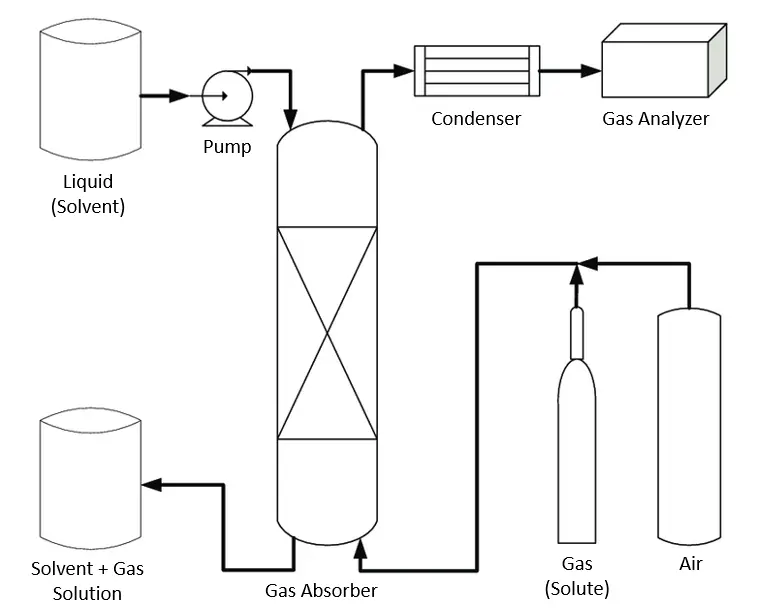
Gas (solute, usually mixed with air) feed enters at the bottom of the absorber column while the liquid solvent is pumped at the top and is distributed through a nozzle or spray. Gas and liquid streams come in countercurrent or co-current contact, allowing the gas solute to dissolve in the liquid solvent. The resulting absorber flue gas is then passed through a condenser and gas analyzer to test the final concentration of the gas components after scrubbing. Flue gas composition will determine if the gas needs to be recycled as feed or can be allowed to be released into the environment.
The solute-rich solvent is recovered at the bottom of the absorber column and subsequently processed in a stripping process to separate the rich gas and recover the solvent. Stripping is just a desorption process to recycle the solvent stream.
To increase mass transfer phenomena, liquid distribution mechanisms (nozzles, spray, etc.) are used to maximize the area that the liquid will cover. Furthermore, column internals (e.g. trays and packings) are installed inside the columns to increase the contact area, thus increasing mass transfer significantly.
Though gas absorption setups are designed ideally, common unexpected issues occur:
Flooding inside the column happens when the velocity of the gas phase is greater than that of the liquid, causing the liquid not to drain correctly across the column internals. Adjusting the gas feed parameters (pressure, feed rate) as well as using the appropriate column internals will help in addressing this issue.
Foaming happens when the liquid expands causing high resistance between the gas and liquid phases. This is primarily caused by impurities present in the gas feed and can be solved by subsequent absorption processes. A thorough analysis and consideration of the feed should be conducted to optimize scrubbing.
Gas absorption (either physical or chemical) is used in gas purification (e.g. removal of pollutants from the exhaust or from flue gas streams that need to be further processed), gas recovery (e.g. recovery of ammonia from air), and in production of gas solutions for various processes (e.g. absorption of CO2 gas in making carbonated beverages). The choice of which shall then depend on the desired product.
In physical gas absorption, the mass transfer, which increases linearly with pressure following Henry’s Law, happens without a chemical reaction. The absorbing liquid is regenerated (desorption) through the reduction of pressure and temperature.
Chemical gas absorption focuses on the reaction between the solvent and the gas (solute) where the solute is selectively dissolved. The solute-rich solution is sent to a stripping column to recover the solvent, while the desorbed gas is compressed to a supercritical condition for storage. Chemical absorption is used commonly in the petroleum industry (removal of impurities in the product stream) and in the chemical industry where a high level of product purity is required.
Gas absorption is the mass transfer of components from gas to liquid while stripping (desorption) is the mass transfer of components from liquid to gas.
The height of an absorption column depends on the feed conditions, solvent to be used, product requirements, and the rate of absorption at equilibrium conditions. In some conditions, column internals also contribute to column sizing. McCabe-Theile Method is used in the calculation of stages as well as column height.
Minimum gas-liquid (MLG) ratio is the least amount of liquid required to dissolve the required amount of solute at a certain temperature and pressure; the liquid flow is expressed as a function of the gas flow rate to be absorbed.