Microfiltration is a pressure driven membrane separation method in which suspended particles are separated from a fluid when passed through a microporous membrane structure. It is called microfiltration because the particles that are retained are around the size of one micron (one thousandth of a millimeter).
Compared to the other pressure driven membrane filtration methods such as ultrafiltration, nanofiltration, reverse osmosis and gas separation, microfiltration utilizes membranes with the largest pore sizes. Microfiltration membranes can be manufactured from a wide variety of materials which can include both inorganic materials (ceramics and metals) or polymer materials such as nylon.
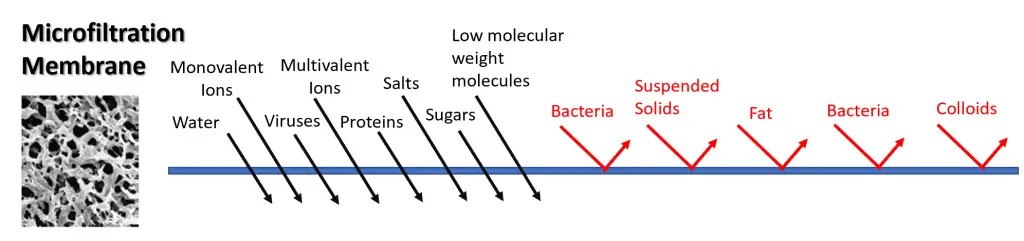
Microfiltration is capable of separating bacteria and suspended solids, however it is also capable of separating fat, bacteria and colloids. It allows water, monovalent ions, multivalent ions and viruses to pass through, as well as low molecular weight molecules, proteins, salts and sugars.
The microporous membrane layer is a thin sheet with open micropore structures that are designed to allow fluids to pass through whilst retaining suspended particles. The mass transport in microfiltration membranes occurs via viscous flow through the micropores.
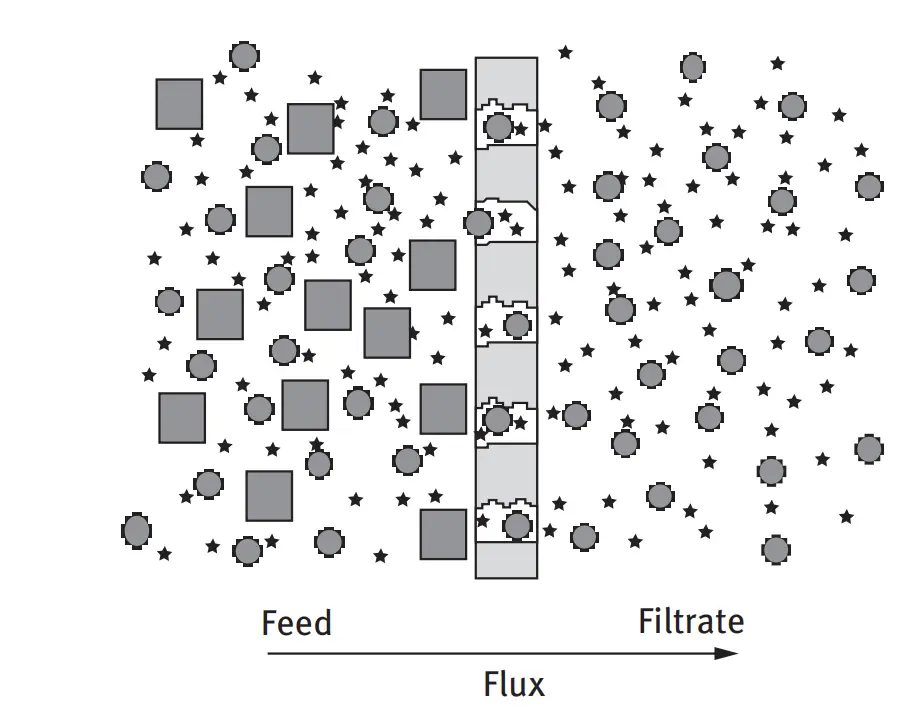
The particles are retained in the microporous membrane in two ways:
This is the most intuitive retention method as particles larger than the pores are physically retained. This is also known as size exclusion. Mechanical retention is typically unaffected by process conditions.
Particles that are small enough to enter the membrane pore structure are able to be retained by entering into the pore walls. This is highly dependent on the membrane materials and the interaction effects (hydrophobic interactions, charge interaction, and internal impaction). Unlike mechanical retention, adsorptive retention can be affected by process conditions (i.e. temperature, viscosity, pH etc.)
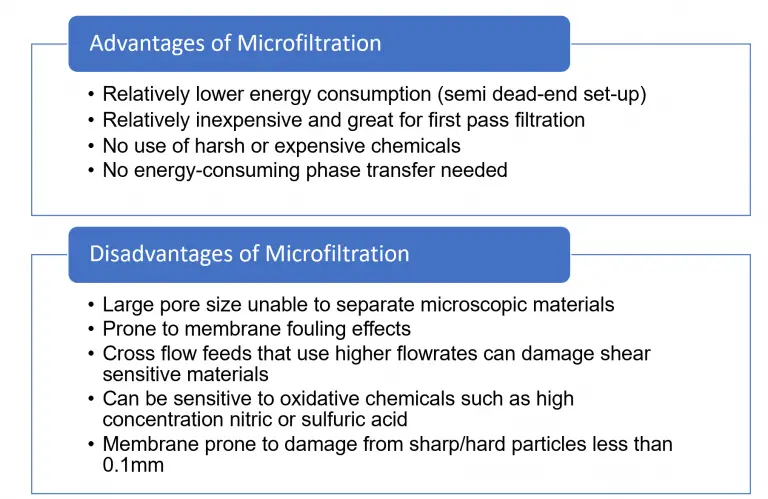
The advantages of microfiltration include:
Some of the disadvantages of microfiltration include:
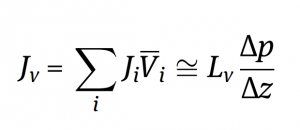
Let’s break this equation down by first understanding what these variables mean.
The total volumetric flux through the microfiltration membrane is the summation of the flux from each individual component in the feed stream multiplied by the respective partial molar volume of each individual component in the feed stream. This equation can also be approximated by the second part of the expression above.
The volumetric flux through the microfiltration membrane is:
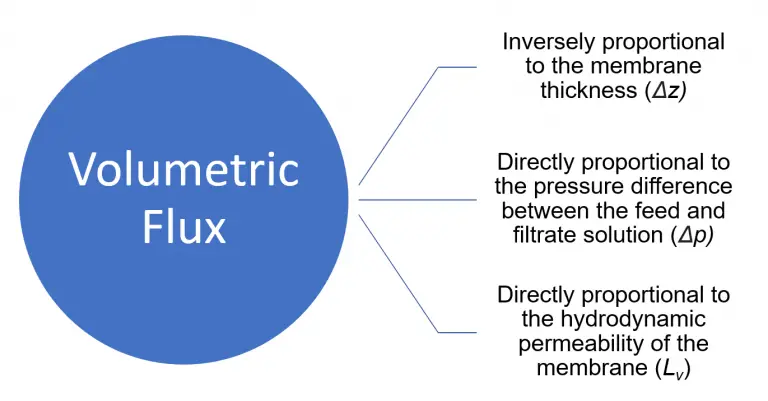
As the hydrodynamic permeability of the membrane (Lv) increases, the membrane is able to allow for an easier passage of components through it, and therefore the volumetric flux increases.
As the pressure difference increases, the force per unit area on the microfiltration membrane increases which forces more fluid to be pushed through the membrane. As a result, the volumetric flux increases.
As the membrane thickness increases, there is more of a barrier that the fluid has to pass through, therefore decreasing the overall volumetric flux.
The equation above is a good approximation for the volumetric flux in a microfiltration system, however, we can look at 2 cases in order to make this equation more precise to the specific structure of membrane.
Defining the new terms here we see:
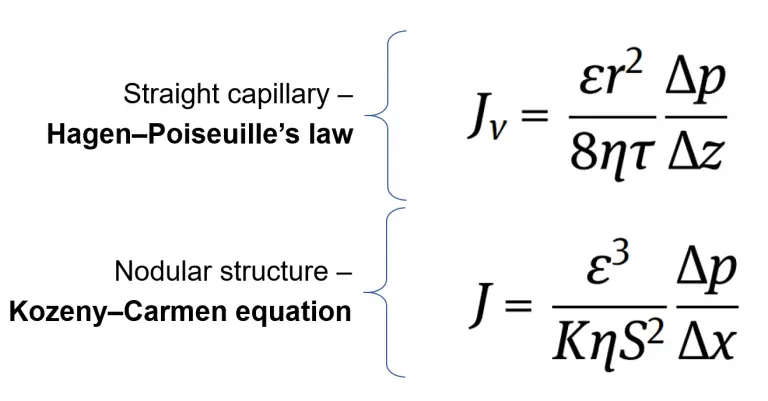
The Hagen–Poiseuille’s law is used if the microfiltration membrane has straight capillaries. This equation adds the porosity, pore radius, viscosity and tortuosity factor to the original volumetric flux approximation equation.
We can see that the volumetric flux is directly proportional to the porosity and the square of the pore radius which makes sense because more fluid can pass through the membrane as there are more pores, and bigger pores.
Likewise, we can see that the volumetric flux is inversely proportional to the viscosity and tortuosity factor. This means that as the viscosity, or resistance to deformation, increases, the flux decreases, and as the tortuosity increases, the flux also decreases. The tortuosity factor is defined as the ratio of the actual pore length to the thickness of the membrane.
The Kozeny–Carmen equation is used in the microfiltration membrane that has a nodular structure. A nodular structure can be characterized by irregularly shaped spherical cells compared across the membrane surface.
We can see that the volumetric flux is directly proportional to the cube of the pore porosity, similar to the Hagen–Poiseuille equation. However, with this nodular structured microporous membrane, the volumetric flux is inversely proportional to the geometry dependent constant, viscosity, and square of the superficial area of spherical particles per unit volume.
Microfiltration as a clarification method is heavily used in the wastewater treatment industry. It is typically a first pass, or pre-treatment method, used to prevent large bacteria, algae or sediment from passing on to some of the finer and more selective pressure driven membrane separation methods.
Clarification is also used in the food and beverage industry. Sparkling apple juice is a great example of microfiltration via clarification at work to produce a clear sparkling drink.
Another common application of microfiltration is to retain microorganisms that are potentially present in the fluid to either reduce the amount of microorganisms or to remove them completely. For example, tissue or cell culture media is typically filtered through membranes to remove bacteria to make sure that the cultures are not contaminated.
Membranes are not used as a filter but as a porous substrate in order to allow cell cultures to grow for attachment dependent cultures. This also allows some access to nutrients through the porous structure.
Cross flow microfiltration occurs when the feed flows parallel to the membrane surface. One of the benefits of a cross flow microfiltration is that the shearing effect caused by the parallel flow reduces fouling and increases the lifetime of the membrane. Another major benefit is that because the flow is parallel to the membrane surface, the feed flow can continuously remove the permeate build up on the surface of the membrane, and the permeate flux drops much more slowly when compared to the dead end filtration method.
Microfiltration cannot remove salts. Reverse osmosis processes can remove salts, and nanofiltration can remove up to 55% of monovalent salt.